Patient-Oriented Research
- NASH CRN
- Twin-Family Study
- DB2
- Registry
- Colesevelam
PI: Rohit Loomba
# 091491
The purpose of the study is to investigate a potential treatment for Nonalcoholic Steatohepatitis (NASH). Nonalcoholic Fatty Liver Disease (NAFLD) covers a range of liver disease progression. When fatty liver disease moves on to a more severe level of liver injury, it is called nonalcoholic steatohepatitis (NASH). NAFLD/NASH is likely the most common liver disease in the United States and is thought to be related to obesity or diabetes. A liver biopsy is usually done to confirm liver disease. The biopsy results may show different amounts of fat, inflammation, and scarring in the liver. NASH can lead to severe liver disease in some patients. In addition to evaluating a potential treatment for NASH, this study will also look at important differences between patients who have NASH and patients who the investigators originally suspected might have NASH— but ultimately did not. This study is sponsored by the company Daiichi Sankyo.
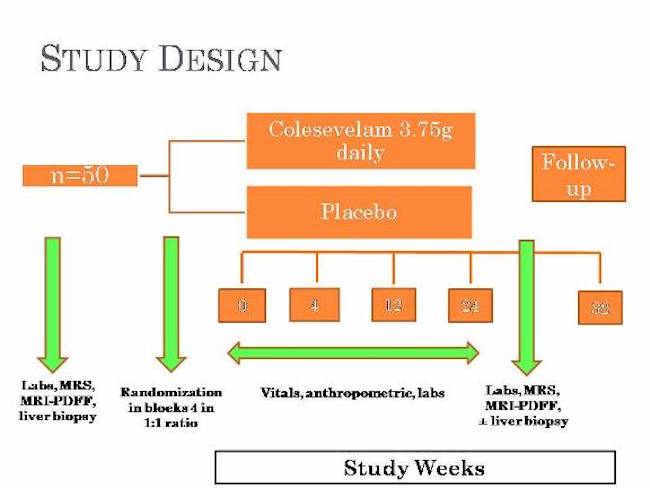
This study is a prospective double-blind randomized controlled trial for treatment of NASH. Participants diagnosed with NASH will be assigned to either the study drug group or the placebo group. Participants who do not have NAFLD/NASH can enter the study as a participant in the control group and will not be assigned any study drug.
At the screening visit, study participants will be asked to come in a fasting state for the blood draw and will receive a physical exam and undergo imaging (magnetic resonance techniques and ultrasound). In addition, participants will be asked questions about alcohol consumption and medication use. A percutaneous liver biopsy will be performed approximately prior to starting Colesevelam in patients with suspected NASH and if they are confirmed to have NASH then they will be asked to continue further in the study. Patients who do not qualify for the treatment part of the study because the screening tests and procedures show that they do not have NAFLD/NASH will be invited to participate in the study as members of the control group.
After enrollment, study participants with NASH to return to the clinic for follow up visits for blood draws and a repeat liver biopsy and MRI scan. Study participants who enroll in the study as part of the control group will be asked to return to the clinic for 5 annual visits during which they will undergo liver imaging.
Note: We have completed the treatment section of this study. We are only enrolling normal controls with no diagnosis of NAFLD/NASH.
Informed Consent Forms:
Publications:
For additional questions regarding this study please contact:
Archana Bhatt
Clinical Research Coordinator
200 West Arbor drive, MC 8413
San Diego, CA 92103-8413
Phone: (619) 471-3915
Fax: (619) 543-2160
Email: abhatt@ucsd.edu