Gupta Lab
- Team
- Research
- Publications
- Products
| Patient Characteristics at time of Colonoscopy with Polypectomy | |
|---|---|
| Colonoscopist Information | |
| Probability patient will have metachronous advanced neoplasia detected at surveillance colonoscopy: %. | |
| This calculator may require JavaScript to work properly. | |
| * The no adenoma group included patients with no adenoma, as well as patients with only a sessile serrated lesion. **ADR is defined as the proportion of colonoscopic examinations performed by a physician that detected one or more adenomas. |
|
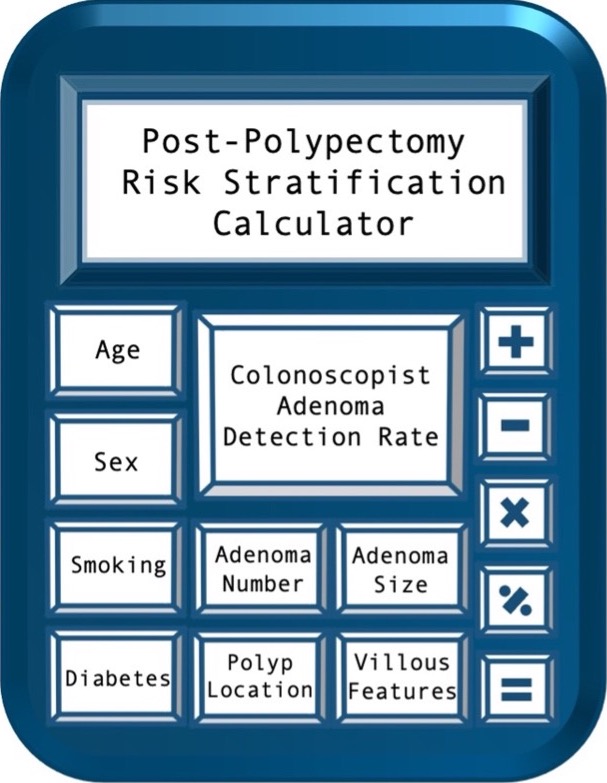
This model was developed as part of a retrospective cohort study of individuals with baseline polypectomy and subsequent surveillance colonoscopy 2004-2016 within the US Department of Veterans Affairs (VA). Clinical factors, polyp findings, and baseline colonoscopist adenoma detection rate (ADR) were considered for the model. Model performance [sensitivity, specificity, and area under curve (AUC)] for identifying individuals with metachronous advanced neoplasia was compared to 2020 US Multi-Society Task Force on Colorectal Cancer (USMSTF) surveillance recommendations. 30,897 individuals were randomly assigned 2:1 into independent model training and validation sets. Increasing age, male gender, diabetes, current smoking, adenoma number, polyp location, adenoma >10 mm or with tubulovillous/villous features, and decreasing colonoscopist ADR were independently associated with metachronous advanced neoplasia. A range of 1.48 to 1.66-fold increased risk for metachronous advanced neoplasia was observed for ADR in the lowest three quintiles (ADR <19.7 to 39.3%) vs. the highest quintile (ADR >47.0%). When the final model selected based on the training set was applied to the validation set, improved sensitivity and specificity over 2020 USMSTF risk stratification were achieved (p<0.001), with an AUC of 0.62 (95% CI: 0.60, 0.64). The calculator above utilizes the variables included in this model. Of note, as of October 2022, the model has not been externally validated in a non-VA cohort. The full report of the study including model development methods is available upon request.
Metachronous advanced neoplasia is defined by the presence of any of the following: colorectal cancer; adenoma 10 mm or larger in size; adenoma with high-grade dysplasia; adenoma with tubulovillous or villous histology.
Reference for the development and validation of the model is our recently published paper titled "Adenoma Detection Rate and Clinical Characteristics Influence Advanced Neoplasia Risk after Colorectal Polypectomy" (Gupta, PI).
Samir Gupta, MD, MSCS, AGAF
s1gupta@health.ucsd.edu