Research at the Llorente Lab
Llorente Lab
The Llorente lab (https://profiles.ucsd.edu/ana.llorenteizquierdo) at the University of California, San Diego, Division of Gastroenterology, Department of Medicine has a keen interest in understanding the complex interactions among the components of the intestinal mucosal barrier and their influence on the onset of liver disease. These components encompass the intestinal immune system, the microbiota, and the intestinal epithelium. These multifaceted interactions play a role in maintaining intestinal homeostasis and are crucially linked to the onset of liver diseases. Our research endeavors are dedicated to elucidating the molecular pathways and factors contributing to liver diseases, with the ultimate goal of identifying therapeutic alternatives.
We investigate the intricate components regulating intestinal homeostasis, such as the intestinal epithelium, microbiome, and immune system, to elucidate their impact on alcohol-associated liver disease (ALD), as well as metabolic dysfunction-associated steatotic and steatohepatitis liver disease (MASLD and MASH) and MASLD and alcohol use disorder (MetALD).

Llorente, Hsu, Hartmann, and Schnabl labs enjoying a picnic at La Jolla Shores, 2024.
We study the implications of the gut-liver axis in preclinical animal models and patient biopsies using cutting-edge microbiomics and state-of-the-art technology, including single-cell RNA sequencing, metabolomics, metatranscriptomics, as well as intestinal and liver organoid cultures. We aim to find innovative strategies for treating liver diseases. The goal of the study is translational with the aim to discover and propose alternative therapeutics.

Llorente, Hsu, Hartmann, and Schnabl labs enjoying celebrating at the Israni Biomedical Research Facility. 2024
Research
Our goal
The Llorente lab has developed a research program with a singular focus: exploring the complexities of the intestinal microbiome, the dynamics of intestinal homeostasis, particularly the intricate interactions with the mucosal immune system, and their implications in the pathogenesis of liver disease. Their research is translational, aiming to find cures for liver diseases.
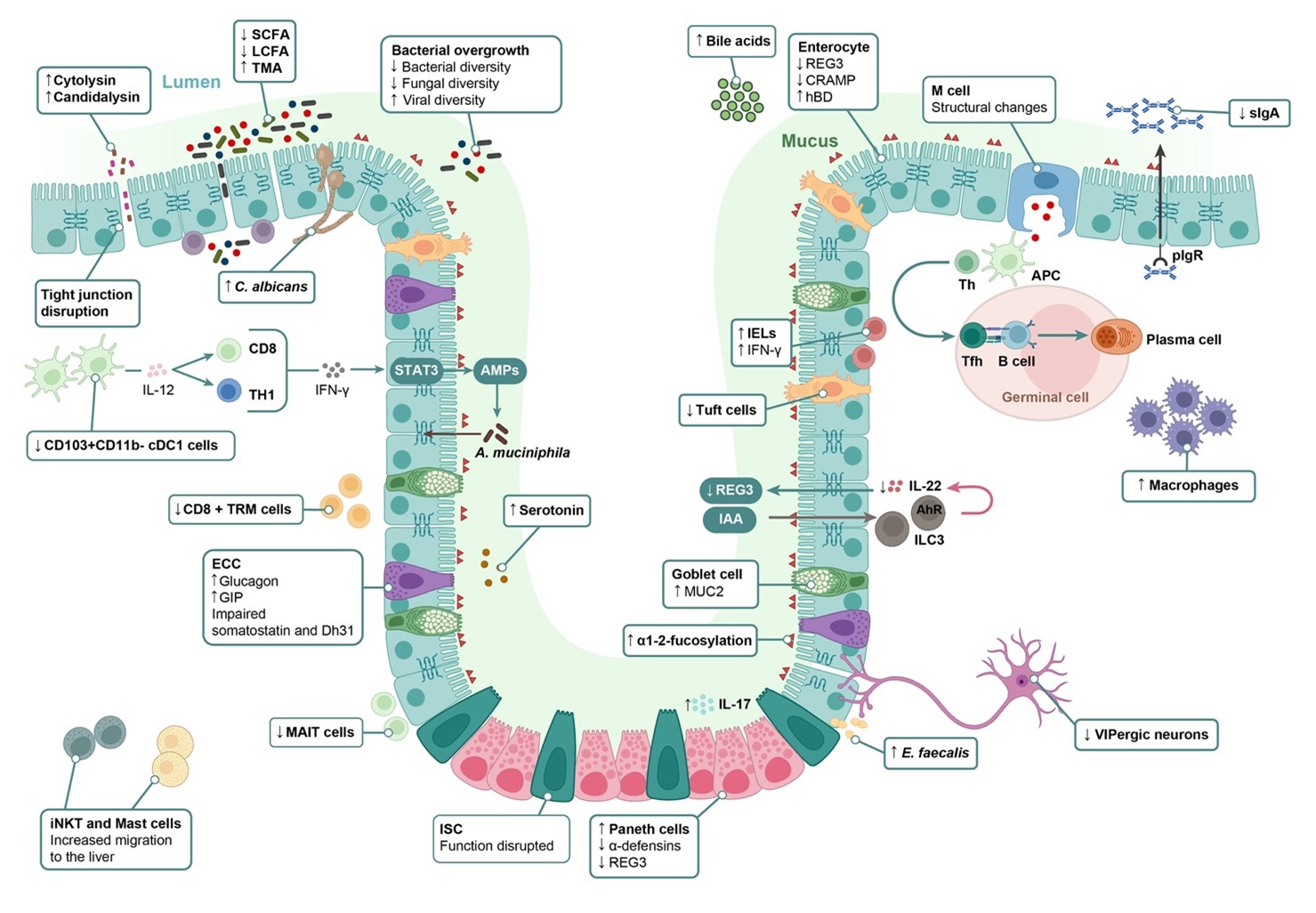
Raya Tonetti F, Llorente C, Hepatology, 2024
Liver disease, much like numerous other health conditions, can disproportionately impact historically marginalized or underrepresented communities. Factors such as socioeconomic status, access to healthcare, genetics, cultural practices, and environmental exposures all contribute to these disparities. Specific liver diseases such as alcohol-associated liver disease (ALD), metabolic dysfunction-associated liver disease (MASLD), metabolic dysfunction-associated steatohepatitis (MASH), and MASLD and alcohol use disorder (MetALD) exhibit a higher prevalence or greater impact within certain underrepresented populations.
The Llorente lab’s research program is designed with the aim of making a positive societal impact. It is estimated that 1.5 billion individuals worldwide suffer from chronic liver disease. Obesity and alcohol consumption have become central liver disease risk factors. Alcohol, as a leading cause of liver disease, is associated with a wide spectrum of hepatic diseases, such as alcohol-associated steatosis, steatohepatitis, fibrosis, cirrhosis, and alcoholic hepatitis, and triggers weakening of the immune system, escalating the susceptibility to bacterial and viral infections.
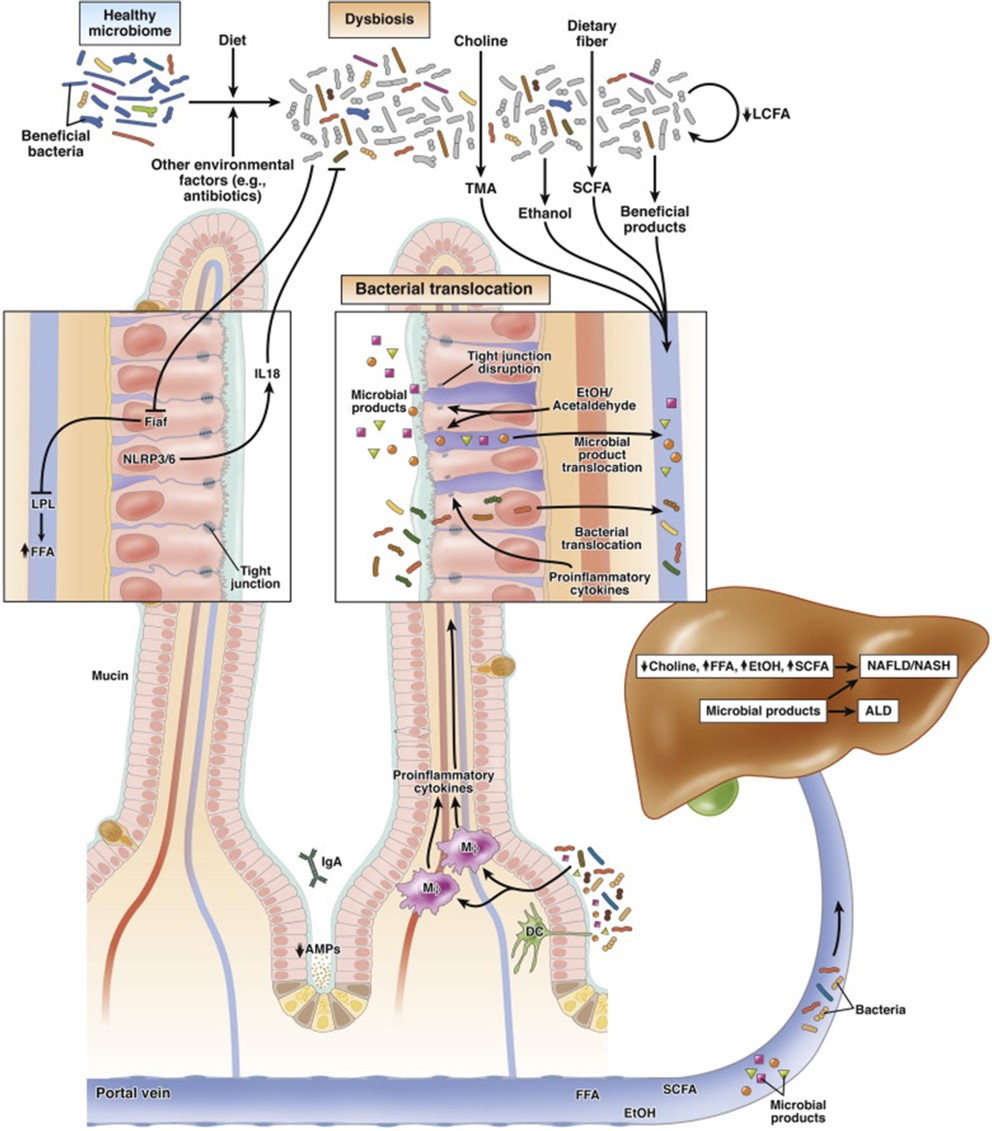
Llorente C, Schnabl B, C Cell Mol Gastroenterol Hepatol, 2015
The influence of microbiota in host physiology plays a central role in regulating the host's metabolism, immune system, integrity of the mucosal barrier, and protection against pathogens. Therefore, microbiota greatly impacts the host’s health and disease. This novel field has opened new avenues in medical sciences to design preventive and interventional therapies to counteract liver diseases. The Llorente lab aims to characterize host-intestinal microbiome interactions to discover alternative therapies to mitigate liver diseases and metabolic diseases.
Specifically, their research is designed to identify:
- Nutritional strategies: By characterizing factors that affect host-microbiome interactions to prevent disease.
- Diagnostic strategies: By depicting markers of disease.
- Molecular targets: To design new drugs to procure a longer and healthier life.
The resulting breakthroughs could not only reduce the healthcare burden but also provide precise strategies to improve patient care and better diagnosis. They also plan to contribute to evolving novel “in vitro” technologies to study molecular pathways that will help to define targets and design new therapies.
Altogether, the Llorente lab’s efforts are intended to benefit society by advancing science and finding resources to procure a healthier life and prevent mortality and disease.
ACCOMPLISHMENTS
The Llorente lab has a great reputation in the field of intestinal microbiome and its impact on chronic liver disease. Our publication in Nature Communications demonstrated that gastric acid suppression contributes to alcohol-associated liver disease in mice and humans. This is mediated by the overgrowth of intestinal Enterococcus and its translocation to the liver.
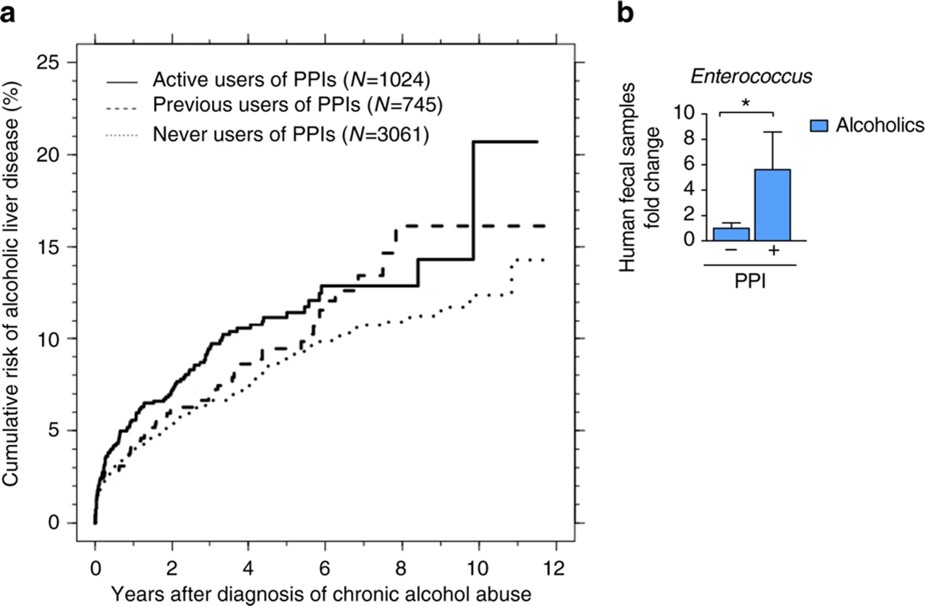
Llorente C, Schnabl B, Nat Commun, 2017
We embarked on a mission to understand the role of Enterococcus in alcohol-related liver disease, culminating in a co-first-author publication in Nature (2022 Impact Factor = 64.8). We reported that alcoholic hepatitis in humans is associated with the presence of a pore-forming toxin called cytolysin produced by Enterococcus faecalis. We showed that cytolytic Enterococcus faecalis is a novel predictor of mortality in alcoholic hepatitis patients.
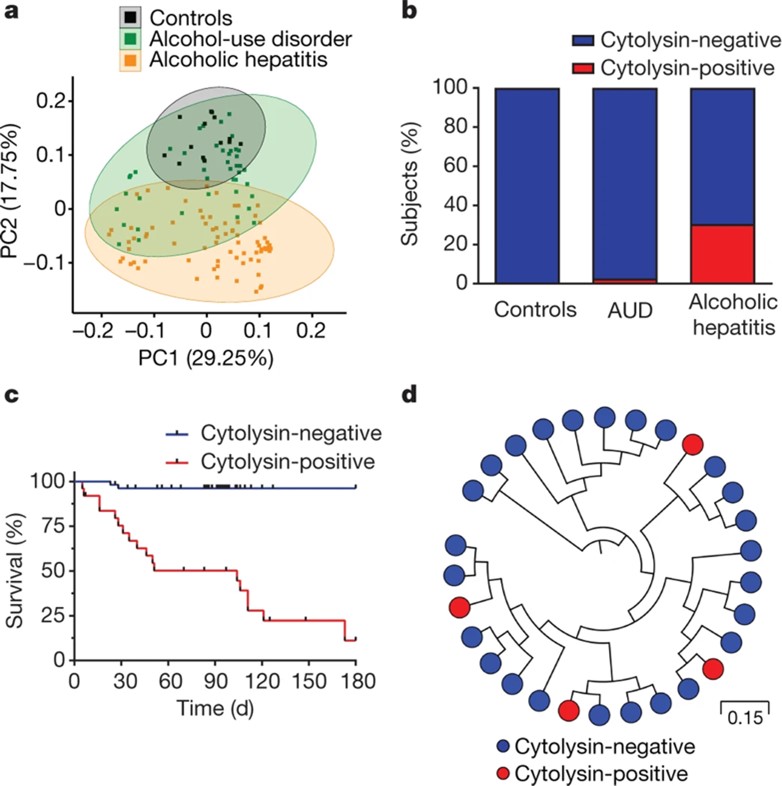
Duan Y*, Llorente C*, Schnabl B, Nature, 2019. (*equal contribution)
We further demonstrated the use of specific bacteriophages as a novel therapeutic strategy to reduce ethanol-induced liver injury in mice.
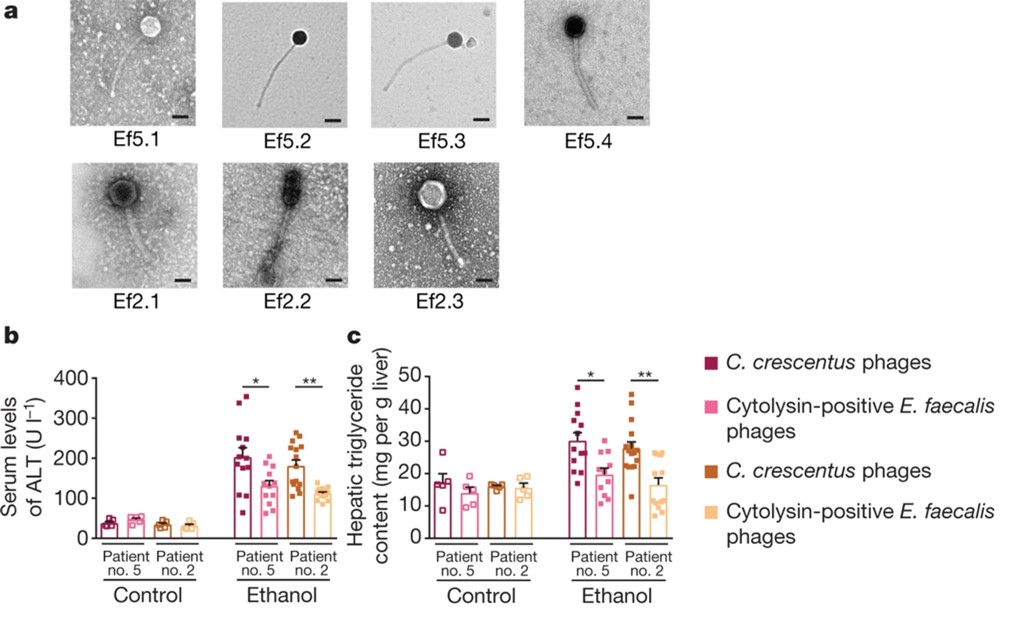
Duan Y*, Llorente C*, Schnabl B, Nature, 2019. (*equal contribution)
The high impact of this publication is evidenced by the numerous editorials that discussed our contribution to the field, which were published in prestigious journals such as Nature, Science, Cell, Nature Reviews Microbiology, Nature Reviews Gastroenterology & Hepatology, Nature Reviews Drug Discoveries, Liver International, Gut, Trends in Microbiology, Translational Research, and Molecular Cell. The article has been accessed 53K times. This article is in the 99th percentile (ranked 225th) of the 346,550 tracked articles of a similar age in all journals and the 97th percentile (ranked 28th) of the 975 tracked articles of a similar age in Nature. This work yielded a patent titled “Biomarker and Treatment of Target for Alcohol Hepatitis” (WO2019191508). The patent describes methods of detecting and monitoring the progression of liver disease, assessing the risk of mortality in humans, and methods of treating liver disease, further emphasizing the impact of our research. Our pioneering research has paved the way for the current commercialization efforts, with the aim of bringing these innovative therapies to clinical trials, where they can make a tangible difference in saving lives.
Our team co-authored a manuscript in Cellular and Molecular Gastroenterology and Hepatology (2022 Impact Factor = 7.2) exploring intestinal α1-2-fucosylation's role in obesity and steatohepatitis using murine models. We found that reducing α1-2-fucosylation protected mice from these conditions by altering the intestinal microbiome and bile acid metabolism. This suggests potential therapeutic avenues for managing obesity and steatohepatitis by targeting α1-2-fucosylation.
Among her achievements, the Llorente lab has pioneered an innovative technique accepted for publication in Cells (2022 Impact Factor = 6), involving the isolation of the myenteric and submucosal plexus from the mouse gastrointestinal tract. Subsequently, these plexuses were co-cultured with small intestinal organoids.
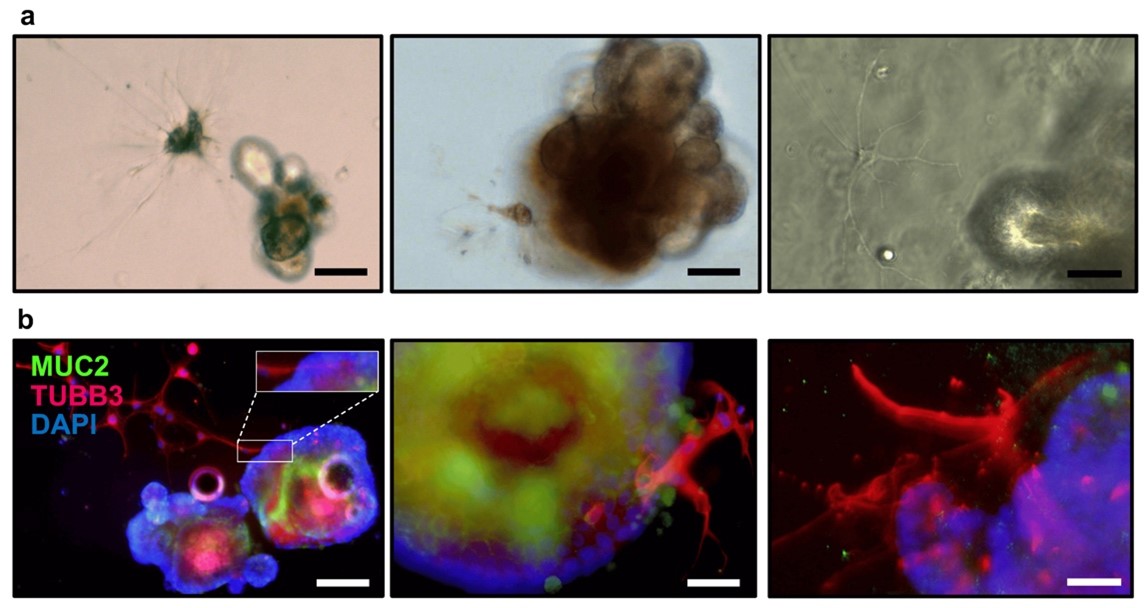 Llorente C, Cells, 2024. (*equal contribution)
Llorente C, Cells, 2024. (*equal contribution)
Currently, the Llorente lab focuses on investigating the role of small intestinal goblet cells as essential factors in maintaining intestinal homeostasis, intending to prevent bacterial translocation and the development of alcohol-associated liver disease.
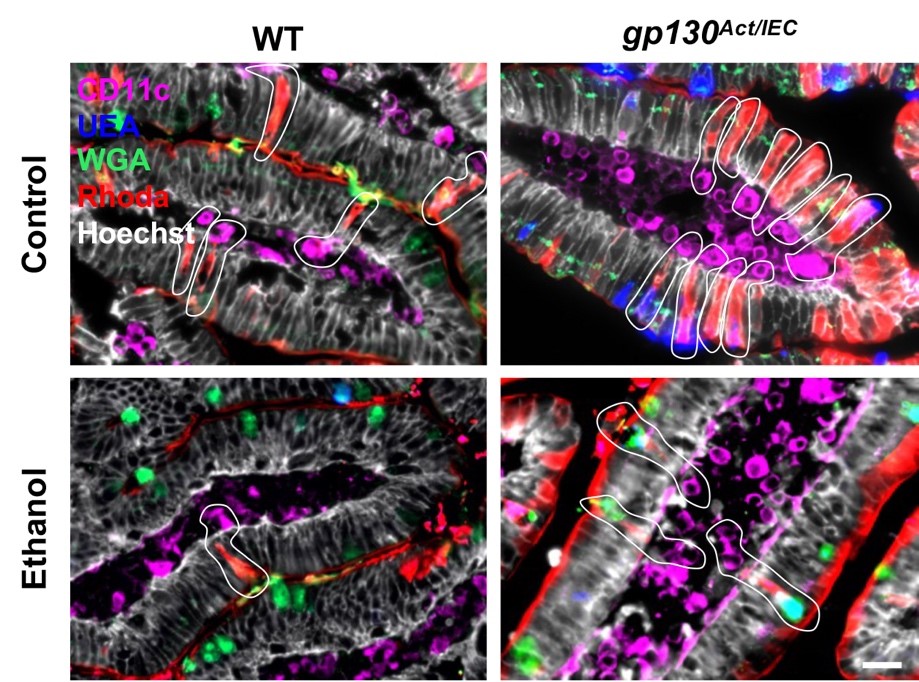
The Llorente lab has published manuscripts in high-impact journals such as Nature in 2019 (2022 Impact Factor = 64.8), Nature Communications in 2017 and 2021 (2022 Impact Factor = 16.6), and Cellular and Molecular Gastroenterology and Hepatology in 2017 and 2022 (2020 Impact Factor = 7.2). Additionally, the Llorente lab has published in Cells (2022 Impact Factor = 6.0), Journal of Hepatology in 2020 (2022 Impact Factor = 25.7), Cell Host and Microbe in 2016 (2022 Impact Factor = 30.3), Hepatology in 2018, 2023, and 2024 (2022 Impact Factor = 13.5), and the Journal of Clinical Investigation in 2017 (2022 Impact Factor = 15.9), among others.
